ISolate
Sperm separation kit designed to quickly produce clean samples, with high concentrations of progressive motile sperm from both healthy and low quality semen.
Further Information
As one of the most cited density gradient sperm separation media, ISolate is the media of choice for laboratories worldwide to produce clean, robust samples with optimal recovery rates. It is supplied ready-to-use as a dual density gradient solution to process the wide variety of semen samples encountered in the laboratory. The kit, which consists of a 50% upper layer and 90% lower layer, support use with a two layer protocol for healthy samples or with a single layer protocol that enables separation of motile sperm from low quality semen samples. ISolate effectively removes cellular contaminants (dead sperm, white cells, and miscellaneous debris) to yield a sample that contains predominantly progressive, motile sperm, capable of fertilizing the oocyte for IUI, IVF and ICSI.
- Formulated with sterile, colloidal suspension of uniform silica particles stabilized with covalently-bound hydrophilic silane in HEPES-buffered HTF
- Highest quality raw materials
- Quality tested by human sperm survival assay
- 90% stock available for custom dilutions
- CE Marked
Quality Control Testing:
- Rabbit pyrogen test
- Sterility by the current USP, CFR or Ph. Eur. Sterility Test
- Sperm Survival Assay
- pH
- Osmolality
Case Study: Focus on Sperm to Achieve Better Pregnancy Rates
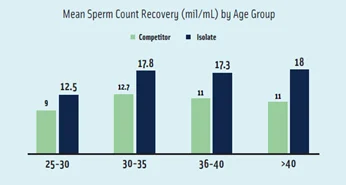
A case study conducted by Dr. Mehta, Lab Director at Queen’s Hospital in Romford, UK, showed a 10% increase in pregnancy rate using ISolate and Multipurpose Handling Medium compared to leading competitor’s media.
Storage: Store at 2-8°C
Shelf Life: 2 years from date of manufacture
| Size | SKU |
|---|---|
| 12x6mL | 99264-12x6mL |
| 2x100mL | 99264-2x100mL |
IFU’s
White Papers & Research Articles
COA Search
Here are the density and density gradients of ISolate.
| Product | Catalog # | Kit Components | Density (g/mL) | Density Gradient (%) |
|---|---|---|---|---|
| ISolate Kit | 99264 | 99257 (Upper Layer – 50%) | 1.06 | 50% |
| 99258 (Lower Layer – 90%) | 1.11 | 90% | ||
| ISolate Stock Solution (90%) | 99275 | N/A | 1.11 | 90% |
To obtain a 40% upper layer from our 50% ISolate Upper Layer, mix 8.0 mL of (upper layer) with 2.0 mL of Modified HTF Medium with Gentamicin - HEPES.
To obtain an 80% lower layer from our 90% ISolate Lower Layer, mix 8.9 mL of lower layer with 1.1 mL of Modified HTF Medium with Gentamicin - HEPES.
We recommend using prepared ISolate gradient within two hours of preparation to avoid the mixing of the gradient prior to use.
ISolate uses a density gradient centrifugation technique as the method of separation. The gradients increase in density from the top to the bottom of the preparation, the sample is gently placed on top of the preparation, and centrifuged. During the process highly motile sperm cells actively move in the direction of the sedimentation gradient. This is in contrast to poorly motile/ immotile sperm cells that do not penetrate the boundaries of the gradient. Upon completion of the centrifugation highly motile sperm cells are enriched in a pellet at the bottom of the preparation1.
References
- Henkel, R. R., & Schill, W. B. (2003). Sperm preparation for ART. Reprod Biol Endocrinol. Nov;14;1:108.
- Hammadeh, M.E., Greiner, P., Rosenbaum, P., Schmidt, W. (2001). Comparison Between Human Sperm Preservation Medium and TEST-Yolk Buffer on Protecting Chromatin and Morphology Integrity of Human Spermatozoa in Fertile and Subfertile Men After Freeze-Thawing Procedure. J Androl. Nov-Dec;22(6):1012-8.
- Wolf, D. P., Patton, P. E., Burry, K. A., & Kaplan, P. F. (2001). Intrauterine insemination–ready versus conventional semen cryopreservation for donor insemination: a comparison of retrospective results and a prospective, randomized trial. Fertil Steril. Jul;76(1):181-5.
- Larson, J. M., McKinney, K. A., Mixon, B. A., Burry, K. A., & Wolf, D. P. (1997). An intrauterine insemination-ready cryopreservation method compared with sperm recovery after conventional freezing and post-thaw processing*. Fertil Steril. July; 68(1), 143–148.
- Medranoa L., Encisob M., Gomez-Torresc M.J., Aizpuruaa J. (2019). First birth of a healthy infant following intra-cytoplasmic sperm injection using a new permeable cryoprotectant-free sperm vitrification protocol. Cryobiology, 2019 Apr;87:117-119.
- Optimization of sperm culture conditions for human ART. Charles L. Bormann and Carol L. Curchoe.
The addition of antibiotics to sperm washing media is at the discretion of the end user. Please reference your own clinic’s protocols when adding antibiotics. For additional details, each laboratory should consult its own laboratory procedures and protocols which have been specifically developed and optimized for your individual medical program. Please reference table below for a complete list of sperm processing products and gentamicin content.
Nexpring Health recommends the following protocol for thawing raw semen and processed sperm.
Yes, ISolate can be diluted with Sperm Washing Medium, or with Modified HTF Medium with Gentamicin - HEPES.
This table details the cryoprotectant, intended use, and use ratio of our sperm preparation media.
| Product | Catalog # | Cryoprotectant | Product Features | Use Ratio |
|---|---|---|---|---|
| Freezing Medium - TYB with Glycerol & Gentamicin | 90128 | 20% v/v egg yolk | For cryopreservation of human semen | 1 to 1 |
| 12% v/v glycerol | ||||
| Refrigeration Medium - TYB with Gentamicin | 90129 | 20% v/v egg yolk | For refrigeration of human sperm up to 96 hours | 1 to 1 |
| Short term storage/capacitation† at room temp | ||||
| Arctic Sperm Cryopreservation Medium | 90170 | 28% v/v glycerol | For cryopreservation and storage of human sperm | 3 to 1 |
† Capacitation at room temperature for approximately two hours.
These are the storage instructions for UNOPENED items.
| Product | Catalog # | Packaging Size | Storage Instructions (unopened product) |
|---|---|---|---|
| Freezing Medium - TYB with Glycerol & Gentamicin | 90128 | 20 x 5 mL | Below -10°C |
| 100 mL | |||
| Refrigeration Medium - TYB with Gentamicin | 90129 | 20 x 5 mL | Below -10°C |
| Arctic Sperm Cryopreservation Medium | 90170 | 12 x 5 mL | 2-8°C |
Here is the shelf life for OPENED items.
| Product | Catalog # | Open Vial Stability | Can product be aliquoted?* |
|---|---|---|---|
| Freezing Medium - TYB with Glycerol & Gentamicin | 90128 | N/A (single use only) | Yes |
| Refrigeration Medium - TYB with Gentamicin | 90129 | N/A (single use only) | Yes |
| Arctic Sperm Cryopreservation Medium | 90170 | 7 days | No |
*If smaller aliquots are desired, the end user may thaw the product and using aseptic technique aliquot into sterile labeled containers and freeze until time of use. Aliquots should be single use and product should not be exposed to repeated freeze thaw cycles.
No, Refrigeration Medium - TYB is intended for short term refrigerated sperm storage. Refrigeration Medium- TYB is typically used to store sperm for up to 96 hours between 2-8°C, transporting semen, and as an aid in sperm capacitation for conventional fertilization.
For freezing sperm we recommend using Freezing Medium - TYB with Glycerol & Gentamicin or Arctic Sperm Cryopreservation Medium.
Both products are intended to be single use and we recommend discarding any excess medium that remains after the procedures are completed. To optimize usage of your product, we recommend thawing the bottle/vial and aseptically aliquoting the media into smaller sterile bottles/vials and stored as directed on the product insert. Do not use aliquots that show a shift in color, particulate matter or any evidence of contamination.
This product is for customers who prefer to freeze sperm without the use of test yolk buffer for long term storage. The product should be used in a ratio of 3:1 (sperm:Arctic).
In general, the recovery rate is around 50% post-thaw when using Freezing Medium - TYB with Glycerol & Gentamicin.
| Fresh | Post-Thaw | |||
|---|---|---|---|---|
| Raw Semen | Freezing Medium TYB | Commercial Medium, Glycerol Only | ||
| Healthy semen (n=25) | Normal morphology (%) | 26.3 ± 7.5 | 22.2 ± 6.4 | 19.4 ± 6.5 |
| Condense chromatin (%) | 92.4 ± 8.5 | 88.7 ± 11.2 | 85.2 ± 12.5 | |
| Motility (%) | 52.8 ± 27.3 | 32.7 ± 19.1 | 29.1 ± 17.1 | |
| Poor quality semen (n=35) | Normal morphology (%) | 11.7 ± 6.1 | 9.3 ± 5.6 | 7.8 ± 4.2 |
| Condense chromatin (%) | 78.9 ± 10.3 | 70.7 ± 10.8 | 64.6 ± 13.0 | |
| Motility (%) | 28.3 ± 22.5 | 13.3 ± 9.9 | 10.7 ± 7.5 | |
We recommend that the TYB freezing medium should be removed prior to IUI either by simple washing with Sperm Washing Medium or Multipurpose Handling Medium - Complete, or if washing thawed raw semen, by density gradient centrifugation with ISolate2,3,4.
References
- Henkel, R. R., & Schill, W. B. (2003). Sperm preparation for ART. Reprod Biol Endocrinol. Nov;14;1:108.
- Hammadeh, M.E., Greiner, P., Rosenbaum, P., Schmidt, W. (2001). Comparison Between Human Sperm Preservation Medium and TEST-Yolk Buffer on Protecting Chromatin and Morphology Integrity of Human Spermatozoa in Fertile and Subfertile Men After Freeze-Thawing Procedure. J Androl. Nov-Dec;22(6):1012-8.
- Wolf, D. P., Patton, P. E., Burry, K. A., & Kaplan, P. F. (2001). Intrauterine insemination–ready versus conventional semen cryopreservation for donor insemination: a comparison of retrospective results and a prospective, randomized trial. Fertil Steril. Jul;76(1):181-5.
- Larson, J. M., McKinney, K. A., Mixon, B. A., Burry, K. A., & Wolf, D. P. (1997). An intrauterine insemination-ready cryopreservation method compared with sperm recovery after conventional freezing and post-thaw processing*. Fertil Steril. July; 68(1), 143–148.
- Medranoa L., Encisob M., Gomez-Torresc M.J., Aizpuruaa J. (2019). First birth of a healthy infant following intra-cytoplasmic sperm injection using a new permeable cryoprotectant-free sperm vitrification protocol. Cryobiology, 2019 Apr;87:117-119.
- Optimization of sperm culture conditions for human ART. Charles L. Bormann and Carol L. Curchoe.
Nexpring Health has not performed studies to confirm this usage and does not recommend the combined usage of its reagents with those from other vendors. However, independent recent studies report usage of MHM-C with other vitrification reagents and protocols5.
References
- Henkel, R. R., & Schill, W. B. (2003). Sperm preparation for ART. Reprod Biol Endocrinol. Nov;14;1:108.
- Hammadeh, M.E., Greiner, P., Rosenbaum, P., Schmidt, W. (2001). Comparison Between Human Sperm Preservation Medium and TEST-Yolk Buffer on Protecting Chromatin and Morphology Integrity of Human Spermatozoa in Fertile and Subfertile Men After Freeze-Thawing Procedure. J Androl. Nov-Dec;22(6):1012-8.
- Wolf, D. P., Patton, P. E., Burry, K. A., & Kaplan, P. F. (2001). Intrauterine insemination–ready versus conventional semen cryopreservation for donor insemination: a comparison of retrospective results and a prospective, randomized trial. Fertil Steril. Jul;76(1):181-5.
- Larson, J. M., McKinney, K. A., Mixon, B. A., Burry, K. A., & Wolf, D. P. (1997). An intrauterine insemination-ready cryopreservation method compared with sperm recovery after conventional freezing and post-thaw processing*. Fertil Steril. July; 68(1), 143–148.
- Medranoa L., Encisob M., Gomez-Torresc M.J., Aizpuruaa J. (2019). First birth of a healthy infant following intra-cytoplasmic sperm injection using a new permeable cryoprotectant-free sperm vitrification protocol. Cryobiology, 2019 Apr;87:117-119.
- Optimization of sperm culture conditions for human ART. Charles L. Bormann and Carol L. Curchoe.
A study by Bormann and Curchoe demonstrated the superior performance of dual-buffer solution of HEPES and MOPS containing Multipurpose Handling Medium - Complete (MHM-C) for key sperm parameters over single buffer controls6.
References
- Henkel, R. R., & Schill, W. B. (2003). Sperm preparation for ART. Reprod Biol Endocrinol. Nov;14;1:108.
- Hammadeh, M.E., Greiner, P., Rosenbaum, P., Schmidt, W. (2001). Comparison Between Human Sperm Preservation Medium and TEST-Yolk Buffer on Protecting Chromatin and Morphology Integrity of Human Spermatozoa in Fertile and Subfertile Men After Freeze-Thawing Procedure. J Androl. Nov-Dec;22(6):1012-8.
- Wolf, D. P., Patton, P. E., Burry, K. A., & Kaplan, P. F. (2001). Intrauterine insemination–ready versus conventional semen cryopreservation for donor insemination: a comparison of retrospective results and a prospective, randomized trial. Fertil Steril. Jul;76(1):181-5.
- Larson, J. M., McKinney, K. A., Mixon, B. A., Burry, K. A., & Wolf, D. P. (1997). An intrauterine insemination-ready cryopreservation method compared with sperm recovery after conventional freezing and post-thaw processing*. Fertil Steril. July; 68(1), 143–148.
- Medranoa L., Encisob M., Gomez-Torresc M.J., Aizpuruaa J. (2019). First birth of a healthy infant following intra-cytoplasmic sperm injection using a new permeable cryoprotectant-free sperm vitrification protocol. Cryobiology, 2019 Apr;87:117-119.
- Optimization of sperm culture conditions for human ART. Charles L. Bormann and Carol L. Curchoe.
Components of cryopreservation media are different for sperms and oocytes/embryos. FUJIFILM Irvine Scientific offers specific media for this application. Please consider Vit Kit-Freeze and Vit Kit-Thaw for oocytes and embryos.